Metastatic High-Risk CSPC LATITUDE Study
In men with metastatic high-risk CSPC
AFTER A MEDIAN FOLLOW-UP OF 52 MONTHS1
Median Overall Survival (OS)
ZYTIGA® + prednisone demonstrated a statistically significant improvement in OS, based on a pre-specified interim analysis, compared to placebos
STUDY DESIGN1-3
- ZYTIGA® + prednisone were evaluated in a phase 3 multicenter, randomized, double-blind, placebo-controlled clinical trial
- Newly diagnosed (≤3 months before randomization) metastatic high-risk CSPC (N=1199)†
- High-risk prognosis was defined as having 2 of the following 3 risk factors:
- Gleason score ≥8
- Presence of ≥3 bone lesions
- Presence of measurable visceral metastasis
- Patients with significant cardiac, adrenal, or hepatic dysfunction were excluded.
- Patients were randomized 1:1 to receive:
- ZYTIGA® orally at a dose of 1,000 mg once daily + prednisone 5 mg orally once daily or
- Placebos
- All the patients received GnRH analogs or had prior bilateral orchiectomy during the trial
- Patients continued treatment until radiographic or clinical disease progression, unacceptable toxicity, withdrawal or death. Clinical progression was defined as the need for cytotoxic chemotherapy, radiation, or surgical treatment for cancer, pain requiring chronic opioids, or ECOG performance status decline ≥3
- Efficacy end points:
- Overall survival (OS)
- Median time to initiation of chemotherapy (secondary)
A major efficacy outcome was overall survival. The pre-specified interim analysis after 406 deaths showed a statistically significant improvement in OS in patients on ZYTIGA® with prednisone compared with those on placebos. Twenty-one percent of patients in the ZYTIGA® arm and 41% of patients in the placebos arm received subsequent therapies that may prolong OS in metastatic CRPC. An updated survival analysis was conducted when 618 deaths were observed. The median follow-up time was 52 months. Results from this analysis were consistent with those from the pre-specified interim analysis. At the updated analysis, 29% of patients in the ZYTIGA® arm and 45% of patients in the placebos arm received subsequent therapies that may prolong OS in metastatic CRPC.
Kaplan-Meier Plot of Overall Survival; Intent-to-Treat Population in LATITUDE Updated Analysis
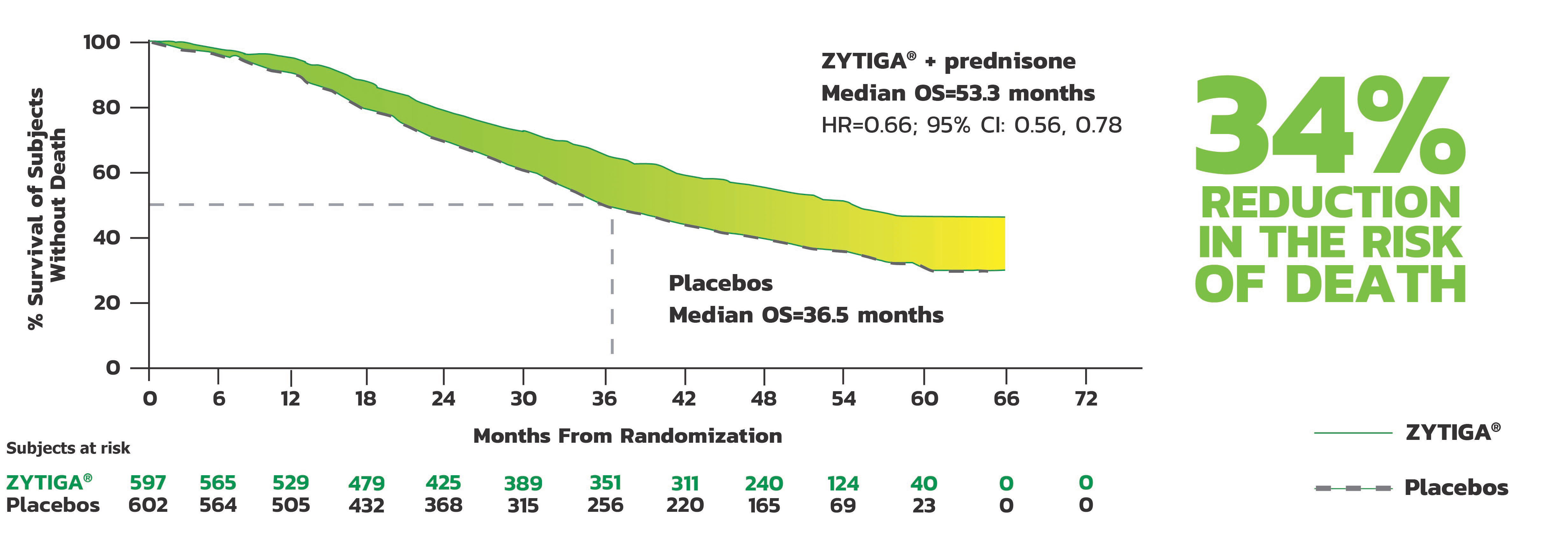
- The major efficacy outcome was supported by a statistically significant delay in time to initiation of chemotherapy for patients in the ZYTIGA® arm compared to those in the placebos arm. The median time to initiation of chemotherapy was not reached for patients on ZYTIGA® with prednisone and was 38.9 months for patients on placebos (HR = 0.44; 95% CI: [0.35, 0.56], P<0.0001).
KEY: ADT = androgen deprivation therapy; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; GnRH = gonadotropin-releasing hormone; HR = hazard ratio.
† Selected exclusion criteria included any prior pharmacotherapy, radiation therapy, or surgery with curative intent for metastatic prostate cancer; the following exceptions were allowed: (1) up to 3 months of ADT (with GnRH analogs or orchiectomy, with or without concurrent antiandrogens) prior to randomization; (2) one course of palliative radiation or surgical therapy administered prior to randomization to treat symptoms associated with metastatic disease. Patients were ineligible if AST and/or ALT ≥2.5X.3
Mineralocorticoid excess - Closely monitor patients with cardiovascular disease. Control hypertension and correct hypokalemia before treatment. Monitor blood pressure, serum potassium, and symptoms of fluid retention at least monthly. The safety of ZYTIGA® in patients with LVEF <50% or NYHA Class III or IV heart failure in COU-AA-301 or NYHA Class II to IV heart failure in COU-AA-302 and LATITUDE was not established.
Adrenocortical insufficiency - Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during, and after stressful situations.
Hepatotoxicity - Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue ZYTIGA® dosing as recommended.
Increased fractures and mortality in combination with radium Ra 223 dichloride - Use of ZYTIGA® plus prednisone/prednisolone in combination with radium Ra 223 dichloride is not recommended.
Embryo-Fetal Toxicity - ZYTIGA® can cause fetal harm and loss of pregnancy. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 weeks after the last dose.
Hypoglycemia - Severe hypoglycemia has been reported when ZYTIGA® was administered to patients with pre-existing diabetes receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide [see Drug Interactions (7.2)]. Monitor blood glucose in patients with diabetes during and after discontinuation of treatment with ZYTIGA®. Assess if antidiabetic drug dosage needs to be adjusted to minimize the risk of hypoglycemia.
Adverse Reactions - The most common adverse reactions (≥10%) are fatigue, arthralgia, hypertension, nausea, edema, hypokalemia, hot flush, diarrhea, vomiting, upper respiratory tract infection, cough, and headache.
The most common laboratory abnormalities (≥20%) are anemia, elevated alkaline phosphatase, hypertriglyceridemia, lymphopenia, hypercholesterolemia, and hypokalemia.
Please see Important Safety Information.
ADVERSE REACTIONS ON THE ZYTIGA® PLUS PREDNISONE ARM THAT OCCURRED IN ≥5% OF PATIENTS WITH A ≥2% ABSOLUTE INCREASE IN FREQUENCY COMPARED TO THOSE ON THE PLACEBOS ARM2
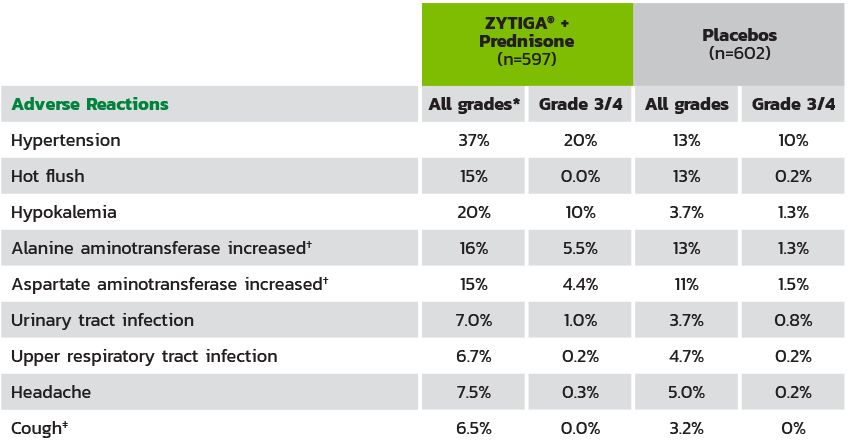
The median duration of treatment was 24 months with ZYTIGA® + prednisone vs 14 months with placebos.2
* Adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.2.
† Reported as an adverse event or reaction.
‡ Including cough, productive cough, upper airway cough syndrome.
References: 1. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE):final overall survival analysis of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. 2. Fizazi K, Tran N, Fein L, et al; for the LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017; 377(4):352-360 3. Data on file. Janssen Biotech, Inc.
